Are the best drugs injected or snorted? No, not those kinds of drugs!: ARS Pharmaceuticals $SPRY
Ten Bagger or Bust Series
Welcome back to the “Ten Bagger of Burst” series where the stock under scrutiny has the capability of providing a 10x return in a relatively short period of time, but also has the potential to go to zero.
So far, out of all of the Ten Baggers or Bust, most are flat, one has doubled, and one has gone to zero. Not the track record I wanted, but certainly the track record I have.
I am proud to be a generalist investor, but it is worth noting that there is a durable advantage that does not get arbitraged away by competition, and that is industry expertise. For what the academic findings are worth, a fund manager or an analyst with experience working in a specific sector on average will beat their sector benchmark.
If I were young enough so that it made sense to pay my dues in a sector to gain expertise, I would choose biotech. It is the highest returning sector over the last forty years, beating even the tech sector. But as a generalist investor looking at a biotech company, I feel a bit like I’m feeling around in the dark trying to unhook the bra strap of my first crush in junior high school.
For the uninitiated, the biotech landscape in the US is a gauntlet of small cap contenders trying to get their pharmaceuticals through the FDA approval process. That process takes years, and approximately $1 billion per drug, and for those without experience in the process, looks a bit like buying lottery tickets. I am reluctant to say that the whole sector is a gamble in the same way that I am reluctant to say that modern art is all hogwash, there seems to be an ecosystem of people in that subculture who understand it and see it differently. I would have very little to offer in analyzing either modern art, or a biotech company that is undergoing the FDA approval process.
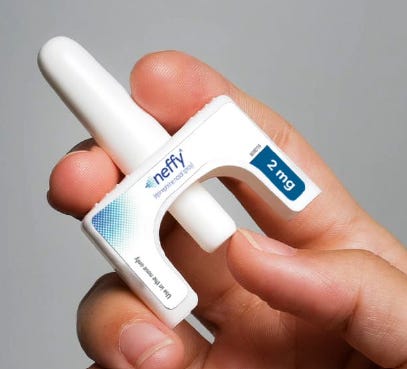
Fortunately, ARS Pharmaceuticals (SPRY) received their FDA approval for Neffy on August 12th. Now their only problem is marketing their epinephrine nasal spray and disrupting the $1 billion annual EpiPen market. For my international readers who don’t live in a world where every third child is deathly allergic to peanuts, emergency injections of epinephrine to prevent anaphylaxis for food allergies or bee stings are not uncommon in the US. I am somewhat surprised to learn that the US is not alone in the rate of severe allergies, although good luck finding a pattern in the map below which makes sense of it. Why do Thailand, Turkey, and Brazil have 1% of children with severe allergies while Finland and China have 9% and 7% respectively? Regardless, that’s a lot of worried mothers who will want to keep a Neffy dose in their purses.
For those countries who agree to the same patent monopoly framework, ARS has a patent on being the only nasal spray epinephrine delivery system until 2038. ARS is a specialist in nasal spray drug delivery, with patents on the device, and a complementary drug, Intravail, which makes the nasal mucous membrane more permeable for drug delivery. Through a licensing agreement, the ARS nasal sprayer is being used by Emergent BioSolutions (EBS) to produce and deliver Narcan, the nasal spray opioid overdose drug.
Should a nasal spray be able to displace the existing EpiPen market dominance? It will if that’s what doctors prescribe, and once informed, doctors should probably switch to prescribing Neffy. Currently half of all patients prescribed for EpiPens don’t even fill the prescription, about 3 million people in the US annually. When polled, about two thirds of those who don’t fill the prescription do so because of concerns about using the needle effectively. Even for people who do carry EpiPens, in about 40% of severe allergic reactions, the EpiPen isn’t used due to concerns about using it incorrectly. Of those who use an EpiPen, about 25% use it incorrectly. If used incorrectly, an injection into bone or a blood vessel as opposed to muscle tissue as intended, can lead to some adverse reactions. Those adverse reactions are much less severe than untreated anaphylactic shock, so the family members or passers by should stop being such neurotic pansies and just use the damn EpiPen. But ARS will be making all efforts to inform doctors of this needle hesitancy, and the rate of adverse reactions due to misuse, and getting them to switch to prescribing Neffy instead of EpiPen.
EpiPen:
There are about 40 million Americans with severe allergies, 20 million diagnosed and under physician care, which has led to 6.5 million epinephrine delivery system prescriptions, of which 3.2 million people filled the prescription. Without the needle hesitancy, would more prescriptions be handed out, and would more prescriptions be filled? If Neffy can take the EpiPen market share in the US, at $199 a dose, that would be a $600 million annual market, not including doses used by first responders, school nurses, or other clinicians who would maintain an inventory for emergencies. Internationally there are opportunities as well, although we all know that drugs are mysteriously more affordable outside of the US. ARS has already begun the process of getting approval in other major markets, with filings within the 2024 calendar year expected in China, Australia, and Japan, all with local partners.
So what kind of price target should I put on ARS Pharmaceuticals? I always get a bit of a chuckle when in a situation of such extreme unknowns, someone puts an estimated return with decimals, or a price target with cents. I have no idea how efficient ARS’ pharmaceutical reps will be at penetrating the existing immunologist or allergist network, and in what timeframe. But I do know that on August 12th, ARS stock price was $10.85, and with the announcement of FDA approval, the price has only moved to $12.98. For a patent until 2038 on something that will probably become commonplace over the next few years, a $1.25 billion market capitalization is probably too low.
The company which owned EpiPen, Mylan N.V. merged with Upjohn in 2020 to create Viatris (VTRS) a $14.5 billion market cap company. They do not release data on what percentage of revenue comes from which product, but ARS claims that this is a $1 billion market. The average price to sales ratio for US listed pharmaceutical companies is between 4x and 6x. So conservatively, going from a $1.25 billion market cap company to a $4 billion market cap company would be about a 3x return. Aggressively, with international markets and increased rates of prescriptions and prescription filling, a 10x return is possible. But most importantly, the only reason why I am writing about a biotech company at all, is that the FDA approval has already been accomplished. All ARS has to do is market and produce their Neffy, which is no simple task, but it is much more of a general business task for a generalist investor to analyze.
Regarding that analysis, ARS has about $200 million in current assets, an amount set aside which they believe is sufficient to get their product to market without the need to raise any additional capital. The chief commercial officer, Eric Karas, has 25 years of experience and was the chief commercial officer for the merchandising of Narcan for Emergent and Adapt Pharma before them. The advertising campaign for Narcan was described as aggressive, and has achieved a market share of 95%, replacing their injection based competition. ARS’ $200 million cash pile which is meant for the commercialization of Neffy came from the 2022 merger with Silverback pharmaceuticals. ARS took 37% dilution for Silverback’s $240 million cash contribution at that time.
ARS hasn’t had any insider buying since the share price was half of where it is today, but that isn’t uncommon, most pharmaceutical companies are riddled with bureaucracy, and capitalists are few and far between. The current President and CEO, Richard Lowenthal, co-founded ARS in 2015 with their chief medical officer Sarina Tanimoto, who is also his wife. Together they still own over 10% of the company, but because of their marital status and the regulations surrounding insider ownership, some sources double count or under count their ownership stake. The ownership stake is hard to calculate as it is split between the two individuals and three separate family trusts, one joint, and one each individual. Some free online sources put the CEO’s ownership stake at 1.5 million shares, rather than the combined 10.6 million shares for the two individuals and their three trusts.
The only red flag I see is the large short interest, which escalated in May when the founding couple sold about $1 million worth of shares. Short interest increased dramatically through July until the August 12th disclosure of the FDA approval. The simplest explanation is that the shorts took large insider selling as a sign of management’s lack of confidence. However, selling 100,000 shares out of 10.6 million, is probably more of an indication of a desire for a bit of a lifestyle upgrade than a loss of confidence in the company. Especially when the March investor presentation claimed that the FDA approval was mostly in the bag after a surprise request for an additional study which was almost certain to pass. (The FDA literally asked them if their product would work if patients had a runny nose. It does.)
My best judgement is that the shorts were misguided to pile in, as opposed to the shorts knowing something important that I have missed. Either way, the path forward for ARS is not entirely clear, acquisitions are common in pharmaceuticals, but their chief commercial officer has a proven track record. I would not be surprised to see ARS get acquired by one of the major pharma companies, nor would I be surprised to see ARS organically and anticlimactically grow their marketshare slowly over the course of the next five years.
With 12 million shares sold short, and such a small price movement after an FDA approval announcement, it’s hard for me to believe that efficient markets have taken in all the available information and that ARS Pharmaceuticals is worth precisely $1.25 billion. I am much more inclined to believe that somebody is wrong, and ARS is worth a punt with a very small portfolio weight, unless you have expertise in biotech.







I subscribed to you early on but admittedly am over subscribed and as prolific as you are, don't read these enough, but this was a great read!
I need to read more of your stuff as I would learn more about valuing companies. You've got a great style. The thesis makes a ton of sense, very logical. I'm a lowly retail investor so opinion doesn't count for much, really just wanted to pass on a compliment. Have a great weekend!
Hey there, I just wanted to say I enjoy your work and would love if you started a YouTube channel or some type of audio talking about your investments and their thesis!! Could gain you a larger following as well